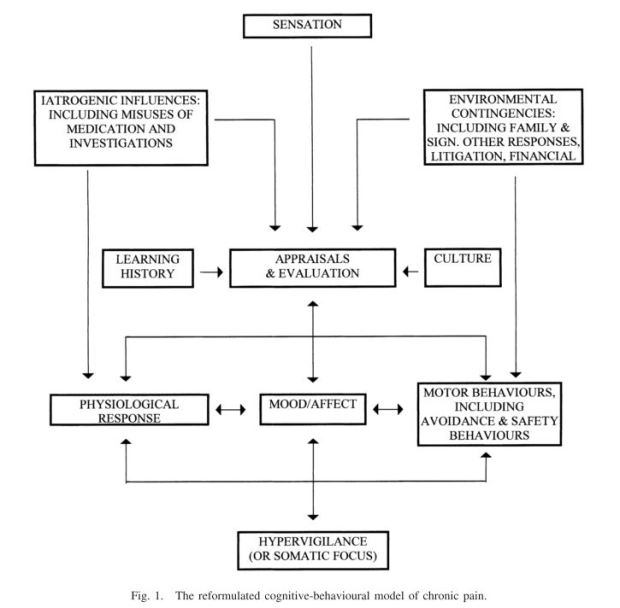
Pain intensity, quality and location are three important domains to consider in pain measurement. And in our kete*of assessment tools we have many to choose from! A current debate (ongoing debate?) in the august pages of Pain (International Association for the Study of Pain) journal shows that the issue of how best to collate the various facets of our experience of pain is far from decided – or even understood.
The McGill Pain Questionnaire (MPQ) is one of the most venerable old measurement instruments in the pain world. It is designed to evaluate the qualities of pain – the “what does it feel like” of sensory-discriminative components, evaluative components, and cognitive-affective components. There are 20 categories in the tool, and these examine (or attempt to measure) mechanical qualities, thermal qualities, location and time. Gracely (2016), in an editorial piece, compares the McGill to a set of paint colour samples – if pain intensity equals shades of grey, then the other qualities are other coloures – blue, green, red – in shades or tints, so we can mix and match to arrive at a unique understanding of what this pain is “like” for another person.
To begin to understand the MPQ, it’s important to understand how it was developed. Melzack recognised that pain intensity measurement, using a dolimeter (yes, there is such a thing – this is not an endorsement, just to prove it’s there), doesn’t equate with the qualities of pain experienced, nor of the impact of previous experiences. At the time, Melzack and Wall were working on their gate control theory of pain, so it’s useful to remember that this had not yet been published, and specificity theory was holding sway – specificity theory arguing that pain is a “specific modality of cutaneous sensation”, while pattern theory held that the experience reflects the nervous systems ability to “select and abstract” relevant information (Main, 2016). So Melzack adopted a previous list of 44 words, carried out a literature review, and recorded the words used by his patients. Guided by his own three dimensional model of pain, he generate three groups of descriptors to begin to establish a sort of “quality intensity scale”. These were then whittled down to 78 words that have been used since, and by used I mean probably the most used instrument ever! Except for the VAS.
There are arguments against the MPQ – I’m one who doesn’t find it helpful, and this undoubtedly reflects that I work in a New Zealand context, with people who may not have the language repertoire of those that Melzack drew on. The people I work with don’t understand many of the words (‘Lancinating‘ anyone?), and like many pain measures, the importance or relevance of terms used in this measure are based on expert opinion rather than the views of those who are experiencing pain themselves. This means the measure may not actually tap into aspects of the experience of pain that means a lot to people living with it. Main (2016) also points out that interpreting the MPQ is problematic, and perhaps there are alternative measures that might be more useful in clinical practice. Some of the criticisms include the difficulty we have in separating the “perceptual” aspects of pain from the way pain functions in our lives, and the way we communicate it, and the MPQ doesn’t have any way to factor in the social context, or the motivational aspects of both pain and its communication.
In a letter to the editor of Pain, Okkels, Kyle and Bech (2016) propose that there should be three factors in the measurement – symptom burden (they suggest pain intensity), side effects (or medication – but what if there’s no medication available?), and improved quality of life (WHO-5). But as Sullivan and Ballantyne (2016) point out in their reply – surely the point of treatment is to improve patient’s lives – “we want to know if it is possible for the patient’s life to move forward again. However it is also important that we do not usurp patients’ authority to judge whether their life has improved” (p. 1574). What weighting we give to, for example, pain reduction vs improved quality of life? I concur. Even the MPQ with all its history doesn’t quite reflect the “what it means to me to experience this pain”.
Did it help? Answering this critical question is not easy. Pain measurement is needed for furthering our understanding of pain, to ensure clinical management is effective, and to allow us to compare treatment with treatment. But at this point, I don’t know whether our measures reflect relevant aspects of this common human experience. Is it time to revisit some of these older measures of pain and disability, and critically appraise them in terms of how well they work from the perspectives of the people living with pain? Does this mean taking some time away from high tech measurement and back to conversations with people?
(*pronounced “keh-teh” – Maori word for kitbag, and often used to represent knowledge)
Gracely, R. H. (2016). Pain language and evaluation. Pain, 157(7), 1369-1372.
Main, C. J. (2016). Pain assessment in context: A state of the science review of the mcgill pain questionnaire 40 years on. Pain, 157(7), 1387-1399.
Okkels, N., Kyle, P. R., & Bech, P. (2016). Measuring chronic pain. Pain, 157(7), 1574.
Sullivan, M. D., & Ballantyne, J. (2016). Reply. Pain, 157(7), 1574-1575.